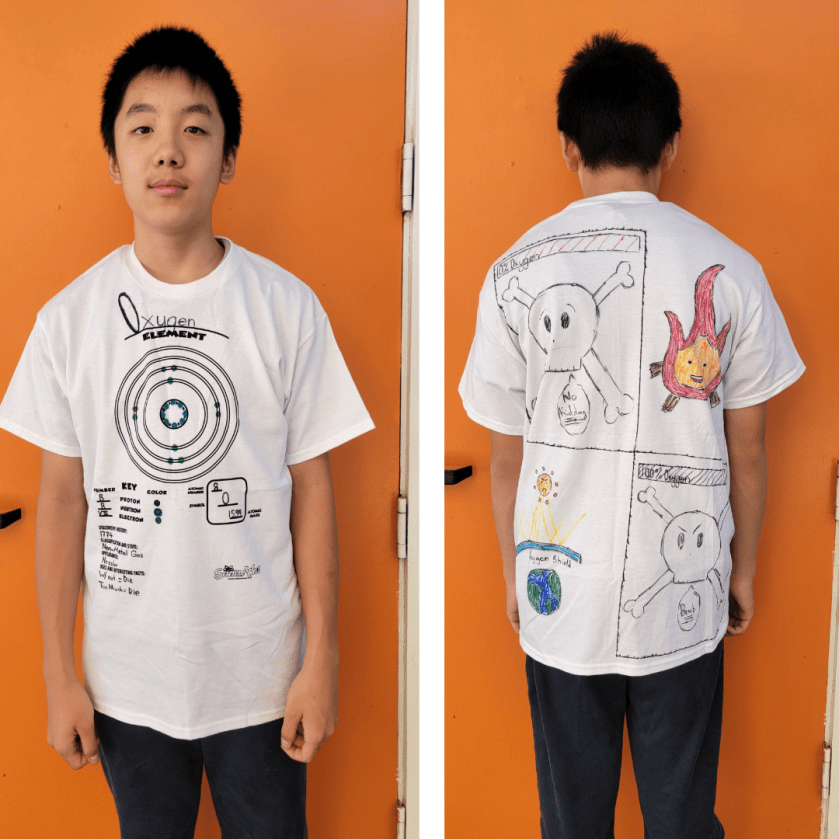
In 8th grade science, students engage in interactive experiments to build a solid knowledge of chemistry. Recently they have been studying chemical bonds. They started exploring ionic and covalent bonds by building on their knowledge of the periodic table and the properties of metals, metalloids, and nonmetals.
Last week, the eighth graders learned how to identify each type of bond, draw representations, recognize bonding types, and correctly create and name chemical formulas. They also investigated polarity within covalent bonds. In covalent bonds, atoms share electrons to form connections. However, because some atoms have more protons (positive charges) in their nuclei, they can pull these shared electrons more strongly, creating a compound with positive and negative ends—similar to a magnet.
The students used food coloring and milk to help them visualize this concept. Milk contains nonpolar fat molecules, while food coloring is polar. Since polar and nonpolar substances don’t mix easily, adding soap, which has both polar and nonpolar ends, acts like a magnet, pulling the food coloring across the milk, and creating a colorful swirl without stirring. The students were fascinated by this vivid demonstration of polarity in action!
#SaklanHandsOn